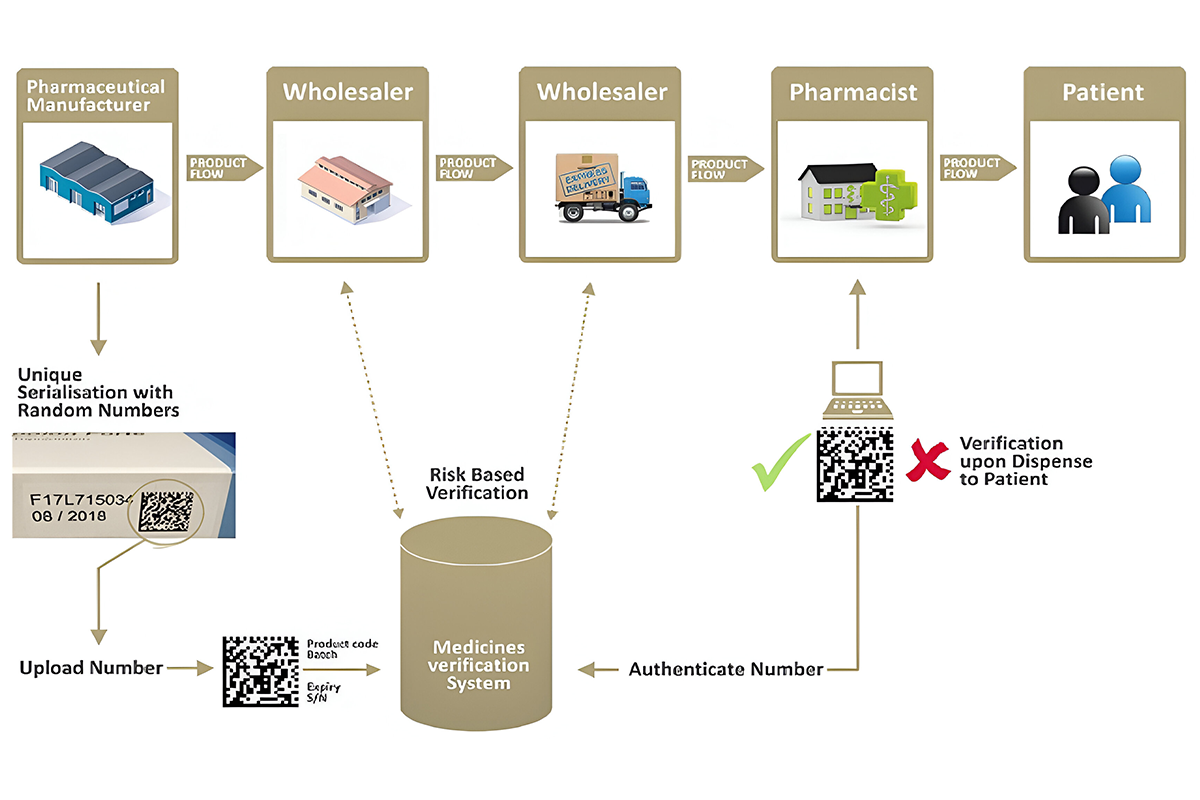
Serialization in the pharmaceutical industry involves assigning a unique digital identity to each medicine pack, typically a 2D barcode encoding product details, batch number, expiration date, and a unique serial number. This data is recorded and tracked throughout the supply chain, providing an auditable trail from manufacturing to patient dispensing. Crucially, this process combats the global threat of counterfeit drugs, enabling ethical manufacturers to ensure patient safety and product integrity. With governments and regulatory bodies worldwide increasingly mandating serialization to secure the drug supply chain.Reliable technology providers offer adaptable systems that help manufacturers implement these complex tracking requirements across diverse packaging lines, ultimately strengthening the fight against fake medicines.
| Feature / Benefit | Other Systems | Sun Teknovation Track & Trace |
|---|---|---|
|
Serialization Type
|
Partial or manual serialization
|
Online & Offline Track & Trace with full 2D barcode & Data Matrix encoding
|
|
Real-Time Verification
|
No real-time checks
|
⏱️
Assigns & verifies unique identifiers in real-time
|
|
Regulatory Compliance
|
Limited / partial 21 CFR
|
📜
21 CFR Part 11 Compliant, GMP-ready
|
|
Machine Control
|
Manual / PLC limited
|
🤖
PLC-controlled with line-level automation
|
|
High-Speed Operation
|
Slower / batch-based
|
🚀
Up to 220 cartons per minute
|
|
Error Handling / Rejection
|
Manual removal
|
💨
Air-based or vacuum rejection for defective items
|
|
Data Storage / Reporting
|
🗂️
Manual logging
|
📊
Inbuilt server with full traceability reports
|
|
Labeling & Tamper Proof
|
Limited
|
🛡️
Anti-tamper labels & double-size labeling supported
|
|
Aggregation Capability
|
Manual linking
|
✅
Supports Manual & Multi 2D Aggregation for units, cases, pallets
|
|
Compact & Flexible Design
|
Large, rigid machines
|
🏗️
Modular, stand-alone, compact design
|